SnoreStop
SnoreStop Anti-Snoring Breathe Easy Formula
Natural Relief for Congestion-Related Snoring.
Description
Description
SnoreStop Breathe Easy Chewable Tablets: Natural Relief for Respiratory-Related Snoring
The SnoreStop Breathe Easy Formula is specifically designed for snorers dealing with respiratory congestion or asthma symptoms. With double the active ingredients of our Maximum Strength Formula and four additional natural decongestants, it opens airways to improve breathing and promote restful sleep.
Key Benefits:
- Powerful Formula: Twice the active ingredients for enhanced effectiveness, plus four natural decongestants to clear airways.
- Better Breathing, Better Sleep: Targets snoring caused by congestion to help you wake up refreshed.
- Easy-to-Use Tablets: Simply chew at bedtime for quick and convenient snoring relief.
- Natural and Safe: Made with FDA-approved, plant-based ingredients, free from known side effects.
Why Choose SnoreStop Breathe Easy Formula?
- Proven Since 1995: The only medically proven over-the-counter natural snoring solution that isn’t a device.
- Hassle-Free Relief: No uncomfortable mouth guards, chin straps, or expensive gadgets—just effective, discreet results.
- Made in the USA: Manufactured in FDA GMP-approved facilities to ensure top-notch quality and safety.
Take control of your nights and enjoy quiet, refreshing sleep with SnoreStop Breathe Easy Chewable Tablets.
FAQs
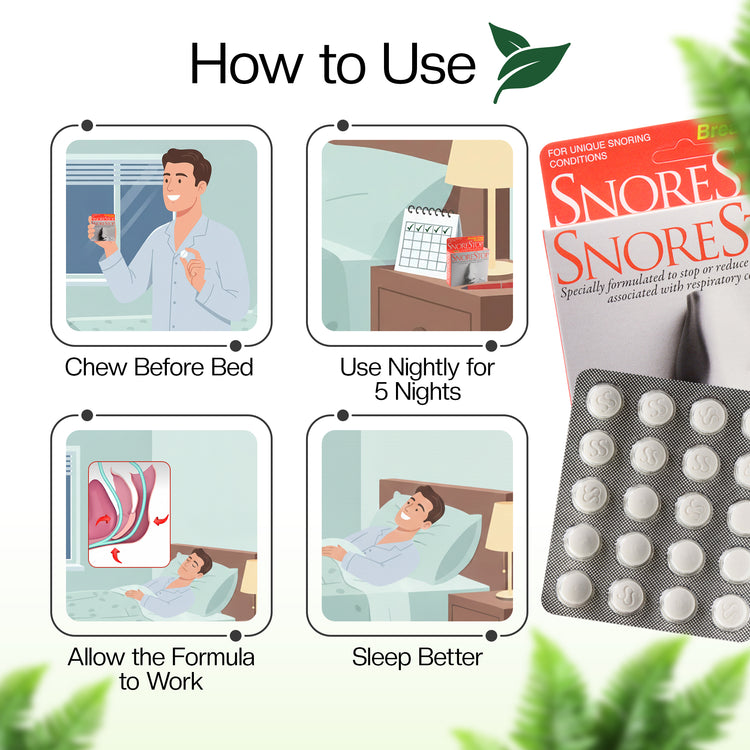
Usage Instructions
Directions for Use:
- Chew one tablet 30 minutes before bedtime and repeat at bedtime.
- Do not take with water; chew or suck tablets.
- 5-7 day treatment course for optimal results.
Treatment Timeline:
- 5-7 Day Course: While some experience results immediately, most users notice improvements within a minimum of 5 nights of consistent use.
Pro Tips for Best Results
-
- Take at bedtime, 30 minutes apart from food, water, or medicine.
- Avoid sedatives, alcohol, or drowsy medication at bedtime.
Experience congestion snoring relief that’s simple, natural, and effective. Reclaim your nights and breathe easier within a week of using the SnoreStop Breathe Easy Formula.
* Individual results may vary. SnoreStop does not treat sleep apnea. Results can depend on a variety of factors including overall health, diet, and other lifestyle factors.
Warnings: Use only as directed. Keep out of reach of children. Do not use it on children under 12 years of age. If pregnant or breastfeeding, ask a doctor before using it. Stop using it and ask a doctor if symptoms do not improve in 7 days.
Ingredients
Ingredients
Ingredients & Purpose:
- Grindelia/Rosin-wood 4X HPUS: helps alleviate symptoms of oppressive breathing with profuse mucous secretion when lying down.
- Laurocerasus/ Cherry-laurel 4X HPUS: helps alleviate symptoms of difficult breathing with a tickling dry cough and snoring while in deep sleep.
-
Zingiber/Ginger 6X HPUS: helps alleviate symptoms of difficult breathing.
-
Arsenicum album/Arsenopyrite 12X HPUS: helps alleviate symptoms of suffocative fits during sleep with sneezing and stopped up nose.
-
Cinchona/Peruviam bark 12X HPUS: helps alleviate symptoms of snoring.
-
Latrodectus mactans/Arachnea 12X HPUS: helps alleviate symptoms of gasping respiration and extreme apnea.
-
Stramonium/Thorn Apple 30X HPUS: helps alleviate symptoms of deep snoring sleep.
-
Belladona 6x HPUS: helps alleviate symptoms of snoring brought on by inflammation of the soft tissues and swelling of the upper respiratory system.
-
Ephedra vulgaris* 6x HPUS: helps relieve the symptoms of snoring that are due to excessive sinus drainage.
-
Hydrastis Canadensis 6x HPUS: helps alleviate the symptoms of snoring that are brought on by thick, tenacious mucus in the sinuses.
- Nux Vomica 6X HPUS: helps constricted pharynx
- Hydrastis Canadensis 6X HPUS: helps decongest swollen tongue
- Kali Bichromicum 6X HPUS : helps relieve stuffy nose
- Histaminum hydrochloricum 6X HPUS: natural antihistamine
Dilution Scale: 6X is equal to one part per million. * Rest easy, SnoreStop is free from ephedrine, pseudoephedrine, or any alkaloids. Your peace of mind is our top priority. SnoreStop has been available on the market since 1995 with a proven safety record. The letters 'HPUS' indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United states.
Inactive Ingredients: Lactose and Magnesium Stearate. Each 300 mg tablet contains 180 mg Lactose. Lactose is a sugar from milk 60% if you do not count lactose as a simple sugar, then you only have 0.003 g of sugar per tablet. If you count lactose as sugar then you have 0.18 g of sugar per tablet (minute amount).
SnoreStop Breathe Easy Formula is prepared in accordance with the Homeopathic Pharmacopoeia of the United States (HPUS). All ingredients are recognized as an official collection of accepted drug ingredients by the Federal Food, Drug and Cosmetic Act and are manufactured in FDA-approved laboratories in the USA. Each ingredient derives either from plant, mineral or protein sources and is present only in harmless microscopic amounts in each dose. None of our ingredients contain any measurable amount of alkaloids, ephedrine or pseudo - ephedrine.
Claims follow requirement in CPG 400.400 and GLP of Sections 502 & 503 of the FDCA (“The Act”) and Part 201 Title 21 of the CFR to include Principle Display Panel of OTC Drug Labeling under 21 CFR 201.62. Uses and claims are taken from various authorized Materia Medica for references. They have not been reviewed by the Federal Drug Administration.
Reviews
Reviews
Couldn't load pickup availability
Share